Renamic features an intuitive user interface and a convenient design for flexible usage
BERLIN--(BUSINESS WIRE)--BIOTRONIK SE & Co. KG announced today the European market launch of their new Renamic implantable cardiac device programmer. Renamic is the latest addition to BIOTRONIK’s new portfolio of external devices launched within only a few months.
The benefits of Renamic have been confirmed in more than 1000 follow-up user tests. Its new user interface has been optimized with improved use of spacing and an intuitive color coding. The touchscreen can either be used with the stylet—or with any other tool, from fingers to ordinary pens. Besides being the smallest programming device in its class, Renamic features special compartments for accessories such as the power cord, so they can be stored within the device during transportation. To ensure maximum compatibility with other systems and technologies, Renamic offers multiple options for data transmission, including Bluetooth®, Global System for Mobile Communications (GSM) and wireless LAN (Wi-Fi®). Furthermore, Renamic is already prepared to accommodate BIOTRONIK SafeSync, which allows for wireless interrogation of the implanted cardiac devices.
“In combination with Reocor, our new external pacemaker, and Reliaty, our new pacing system analyzer, Renamic completes a rapid succession of brand new external device releases,” stated Marlou Janssen, Vice President, Global Marketing and Sales, Cardiac Rhythm Management, BIOTRONIK. “Renamic starts a new era for convenient programming and follow-up of implantable cardiac devices and improves daily clinic efficiency together with BIOTRONIK Home Monitoring® and EHR DataSync®. Renamic once again exemplifies BIOTRONIK’s focus on developing high quality solutions for advanced patient management.”
The programmer is used for initial programming, reprogramming and testing of implantable pacemakers, implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices after implantation. By conducting all measurements and adjustments, Renamic helps clinicians to optimize individual patient therapy.
“The new Renamic programmer is the result of intensive collaboration between engineers and clinicians and is optimally suited for the requirements in daily clinic routines,” says Dr. Heiner Vontobel, Head Cardiologist at Zuercher Oberland Healthcare, Switzerland. “The device’s easy handling and simple programming help to increase my clinic efficiency. Plus, the device’s robust design makes it very reliable.”
About BIOTRONIK SE & Co. KG
As one of the world’s leading manufacturers of cardiovascular medical devices, with several million devices implanted, BIOTRONIK is represented in over 100 countries by its global workforce of over 5,600 employees. Known for having its finger on the pulse of the medical community, BIOTRONIK assesses the challenges physicians face, and provides the best solutions for all phases of patient care, ranging from diagnosis to treatment to patient management. Quality, innovation and reliability define BIOTRONIK and its growing success, and deliver confidence and peace of mind to physicians and their patients worldwide.
More information: www.biotronik.com
Upon publication, please provide us with a copy.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6633847&lang=en
Contacts
BIOTRONIK SE & Co. KG
Sandy Hathaway
Senior Director, Global Communications
Tel. +49 (0) 30 68905 1602
Email: sandy.hathaway@biotronik.com

BIOTRONIK's premium quality external device portfolio: Renamic, Reliaty and Reocor (Photo: Business Wire)
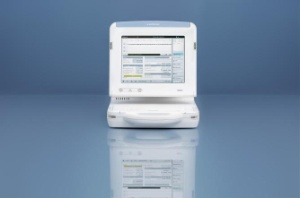
BIOTRONIK's Renamic: the industry's smallest programmer for cardiac devices (Photo: Business Wire)




