TOKYO & BERLIN--(BUSINESS WIRE)--BIOTRONIK, a leading manufacturer of implantable cardiac devices and pioneer of wireless remote monitoring technologies, announced today the Japanese market release of Renamic, their next-generation programmer for implantable devices used in cardiac rhythm management (CRM).
"The growth of cardiac electrophysiology in Japan is significant, and BIOTRONIK is committed to investing in support of this high potential market", commented Dr. Werner Braun, Managing Director, BIOTRONIK. "BIOTRONIK has put great effort into expediting the Japanese market access for Renamic, in order to ensure that our customers have the very latest in new programming technology", continued Dr. Braun.
Renamic, the newest addition to BIOTRONIK's portfolio of external devices, is used for programming, reprogramming and testing of implantable pacemakers, implantable cardiac defibrillators (ICDs) and cardiac resynchronization therapy (CRT) devices.
The CRM market in Japan is estimated at approximately one billion dollars (USD), representing nearly 10% of the global market. BIOTRONIK has a long-standing history in Japan which started in the late 1970s. In order to provide further advanced service and support to its customers, BIOTRONIK began establishing direct Japanese operations in 2003, and has since seen a tremendous expansion of its business. Market share has strongly developed especially in the ICD segment, due to strong customer adoption of the premium quality, state-of-the art Lumax 540 series of devices and BIOTRONIK Home Monitoring®.
The launch of the Renamic is important to the Japanese CRM market because this new generation programmer offers significant advantages with regards to helping optimize in-clinic patient care and cardiac implantable device function. The new device's features provide valuable benefits, such as:
- An intuitive user interface optimized to increase the efficiency of patient follow-up.
- A tested and proven printer from a renowned printer manufacturer.
- A retractable and adjustable touchscreen for optimized viewing and handling.
Easy to handle for efficient and versatile usage, the Renamic's new user interface has been optimized with improved use of spacing and an intuitive color-coding. The touchscreen can either be used with the stylus pen or with any other tool.
"We are pleased to be able to provide Renamic for cardiac clinical teams in Japan. Its easy handling will improve the user's experience as well as potentially save time and cost", commented Jeffrey Annis, Managing Director, BIOTRONIK Japan. "Renamic starts a new era for convenient programming and follow-up of BIOTRONIK cardiac devices and improves daily clinic efficiency."
About BIOTRONIK SE & Co. KG
As one of the world's leading manufacturers of cardiovascular medical devices, with several million devices implanted, BIOTRONIK is represented in over 100 countries by its global workforce of over 5,600 employees. Known for having its finger on the pulse of the medical community, BIOTRONIK assesses the challenges physicians face, and provides the best solutions for all phases of patient care, ranging from diagnosis to treatment to patient management. Quality, innovation and reliability define BIOTRONIK and its growing success, and deliver confidence and peace of mind to physicians and their patients worldwide.
More information: www.biotronik.com
Upon publication, please provide us with a copy.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6868580&lang=en
Contacts
BIOTRONIK SE & Co. KG
Sandy Hathaway
Senior Director, Global Communications
Tel. +49 (0) 30 68905 1602
Email: sandy.hathaway@biotronik.com
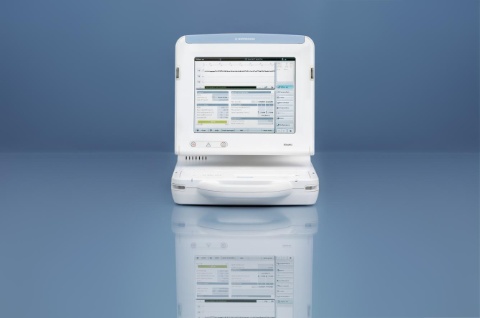
Renamic - external device (Photo: Business Wire)




