DANVERS, Mass.--(BUSINESS WIRE)--NeuroLogica Corporation announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the world's first portable, full body, multi-slice CT scanner, the BodyTom™. (www.NeuroLogica.com)
The BodyTom™ is the newest offering in NeuroLogica’s portable computed tomography imaging line. BodyTom™ is a completely portable, full body, 32 slice CT that boasts an impressive 85cm gantry and 60cm field of view (FOV). The battery powered BodyTom™ can be easily transported from room to room, similar to the widely used portable chest X-ray systems. BodyTom™ is DICOM 3.1 compliant and compatible with all PACS, surgical navigation, electronic medical records, and planning systems. Its unique capabilities provide high quality CT images wherever needed, including the clinic, ICU, OR and Emergency/Trauma Department.
"Our goal with the BodyTom™ is to deliver high quality CT imaging in a completely portable package that is capable of scanning an entire patient’s body. NeuroLogica's development of this globally beneficial product is testimony of our company’s commitment to conceive, design, engineer, manufacture, and deliver innovative, lifesaving, imaging technologies that benefit both clinicians and patients," said Dr. Colin McDonald, Chief Medical Officer of NeuroLogica Corporation.
"BodyTom™ is a revolutionary product. Bringing high resolution imaging technology to the patient rather than moving patients to obtain this kind of vital information for a broad range of applications has to be the future." said Dr. Howard Yonas, Chief of Neurosurgery at University of New Mexico Medical Center. "The ability to bring such a powerful and high quality 32 slice CT scanner into the operating room for intra-operative scanning should have a significant impact on improving care and surgical outcomes.”
"BodyTom™ marks the beginning of a new era in CT imaging," said Dr. In Sup Choi, Medical Director of Interventional Neuroradiology at Lahey Clinic. "Multi slice CT imaging is no longer bound to one room in the hospital. Whether it's in an OR, ED, or ICU, radiologists will be able to scan patients in a more clinically beneficial environment by taking the CT scanner to the patient."
"BodyTom™ represents a significant step forward in the ability of medical organizations to bring the latest CT imaging technology to developing countries such as Haiti," said Dr. Jeffrey B. Mendel, Chief Radiology Advisor to Partners in Health, Mirebalais Hospital, Haiti. "At the moment, NeuroLogica’s portable small bore CereTom® CT, is one of the only functioning CT scanners in Haiti. The addition of the BodyTom™ will greatly expand imaging capabilities in Haiti, improving the diagnosis and treatment of a wide range of medical conditions."
The BodyTom™ is commercially available in 2011.
About NeuroLogica:
NeuroLogica Corporation (www.NeuroLogica.com) specializes in the design and manufacture of cutting edge imaging equipment that is easy to use and brings the power of imaging to the patient wherever they may be. The company's mission is to bring high quality medical imaging to all people regardless of where they live. NeuroLogica`s CereTom® CT is FDA registered and the quality system is certified to ISO 13485:2003 and ISO 9001:2008 with Canadian Medical Device Amendments. NeuroLogica`s CereTom® CT is CE marked (European Conformity) for distribution in the European Union and European Economic Area.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6665315&lang=en
Contacts
NeuroLogica
Benjamin Powell, +1 978-564-8576
bpowell@neurologica.com
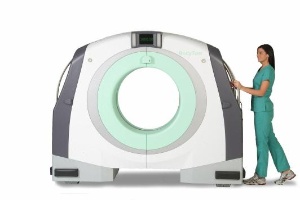
NeuroLogica Corporation announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the world's first portable, full body, multi-slice CT scanner, the BodyTom(TM). (Photo: Business Wire)




