Thin strut PRO-Kinetic Energy bare metal stent with passive PROBIO coating showed excellent results in a 1,000 patient all-comers registry
BUELACH, Switzerland--(BUSINESS WIRE)--BIOTRONIK AG announced that the six-month results of the ENERGY Registry were presented last week by Prof. Raimund Erbel at the Transcatheter Cardiovascular Therapeutics (TCT 2011) conference. This all-comers registry is investigating the safety and clinical performance of the PRO-Kinetic Energy stent system in 1016 patients, including a subanalysis with small vessels, diabetic and elderly patients.
The ENERGY registry was conducted in 49 sites in 10 countries with patients who received a PRO-Kinetic Energy coronary bare metal stent. The study population included a high number of patients with acute coronary syndrome (46%), almost 30% elderly patients (≥75 years), as well as highly complex lesions (39%). The primary endpoint was six-month major adverse coronary events (MACE) defined as a composite of cardiac death, clinically driven target lesion revascularization (TLR) and myocardial infarction.
At six months, the PRO-Kinetic Energy stent system demonstrated excellent clinical outcomes, with a MACE rate of only 4.4%. Cardiac death, myocardial infarction, target lesion revascularization rates were 0.6%, 1.3% and 2.5%, respectively. Only two cases of stent thrombosis (0.2%) were seen. Also, in the small vessel (≤2.75 mm) population, the very low number of events was confirmed with a MACE rate of 6.4% and a TLR of only 2.8%.
"For the treatment of coronary artery lesions, it is crucial to have a bare metal stent available that is not only highly deliverable, but also safe and effective in a broad patient population-including elderly patients and those presenting with acute coronary syndrome, stated Prof. Raimund Erbel, principal investigator from the West German Heart Center in Essen, Germany. "These six-month results are excellent."
Alain Aimonetti, Vice President of Marketing and Sales at BIOTRONIK AG added, "We are delighted that the ENERGY Registry supports our belief that the PRO-Kinetic Energy stent is an excellent platform. For years we have invested in continued development in our stent platforms, and these outstanding results are a testament to our dedication."
The PRO-Kinetic Energy is a thin strut (60μm), cobalt chromium bare metal stent, completely sealed with a thin layer of amorphous silicon carbide, PROBIO. This passive coating reduces the interaction between the metal stent and the surrounding tissue, which makes the stent more hemocompatible.
About BIOTRONIK SE & Co. KG
As one of the world's leading cardiovascular medical device companies, with several million implanted devices, BIOTRONIK is represented in over 100 countries with its global workforce of more than 5100 employees. Known for having its finger on the pulse of the medical community, BIOTRONIK assesses the challenges physicians face, and provides the best solutions for all phases of patient care, ranging from diagnosis to treatment to patient management. Quality, innovation and reliability define BIOTRONIK and its growing success-and deliver confidence and peace of mind to physicians and their patients worldwide.
More information: www.biotronik.com
Upon publication, please provide us with a copy.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=50068299&lang=en
Contacts
BIOTRONIK AG
Ellen Ko, + 41 (0)44 864 51 11
Team Manager, Global Coronary Marketing
ellen.ko@biotronik.com
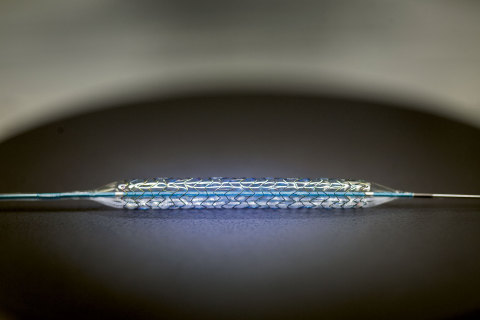
BIOTRONIK PRO-Kinetic Energy Stent-System (Photo: Business Wire)




