BEIJING--(BUSINESS WIRE)--Biocytogen Pharmaceuticals (Beijing) Co., Ltd. (“Biocytogen”, HKEX: 02315) today announces the official formation of two major business divisions to represent the company’s product, service, and asset portfolio. Over the last decade, Biocytogen has developed several technology platforms to streamline the entire drug development process, including generation of specialized (humanized) animal and cell model products, preclinical pharmacology evaluation, and most recently, fully human antibody discovery and clinical development. With the company’s expanding initiatives focused on antibody drug development, the company has officially established two business divisions, with one focusing on animal models and preclinical services, and the other focusing on the discovery and development of novel antibody-based drugs for out-licensing and partnerships.
Biocytogen’s preclinical platforms include custom gene-editing services, generation of off-the-shelf humanized and/or immunodeficient animal and cell model products, and preclinical pharmacology services. These innovative products and services enable efficient evaluation of novel anti-human therapeutics, especially biologics. To better showcase these business lines, and to enhance the brand image of the company’s preclinical models and services, the company will integrate these major platforms under the “BioMice™” sub-brand. As one of the leaders in humanized animal model production, the establishment of the BioMice sub-brand for Biocytogen’s preclinical model and service division will enhance global awareness of the central role of the company’s animal model products in biologic drug discovery.
Biocytogen’s other business division, which harnesses its proprietary fully human antibody RenMice® (RenMab®, RenLite®, RenNano®, RenTCRm™, RenTCR™) platforms to discover novel antibody therapeutics for out-licensing and/or co-development, will be announced at a later date.
BioMice Introduction
With these divisions in place, BioMice products and services will encompass the following:
Gene editing services:
Biocytogen’s gene targeting team provides quality gene-editing services to deliver custom BioMice strains to clients, using ESC/HR (ESC, Embryonic Stem Cell; HR, Homologous Recombination) and EGE™ (Extreme Genome Editing) technologies. Several years ago, the company independently established stem cells on the C57BL/6 background and developed a culture system to keep them totipotent after nearly 100 passages, so that they could be more easily used to obtain adult mice. EGE™ is a CRISPR/Cas9-based gene-editing technology that increases the efficiency of gene knock-in by nearly 20-fold after independent R&D and optimization. In addition, this technology enables the precise editing of DNA sequences at almost any genomic site. To date, approximately 4300 customized gene editing projects have been delivered to customers.
Gene-edited animal and cell model R&D/production/sales:
To date, more than 2900 gene-edited BioMice animal and cell models have been developed for preclinical drug discovery and pharmacology evaluation, and it is expected that 200-300 new models will enter the market each year. These models can be divided into 3 categories:
- Animal and cell models with humanized drug targets, including immune checkpoints (such as PD-1 and CD3E), cytokines and/or cytokine receptors (such as IL-4/IL-4R and IL-33), GPCRs (such as CCR8), TAAs (such as HER2 and TROP2), etc.
- Severely immunodeficient B-NDG™ mice and second-generation B-NDG™ models for human immune system reconstitution, including B-NDG B2m KO mice plus for prolonged experiments by delaying the onset of GvHD, B-NDG hIL15 mice for better human NK cell reconstitution, and B-NDG hTHPO mice that do not require radiation;
- Target gene knock-out mice, disease models, aged mice and other tool mice.
Preclinical pharmacology services:
Taking advantage of the company’s leading position in the generation of innovative animal models, Biocytogen established comprehensive in vivo and in vitro pharmacology platforms to perform drug efficacy evaluations, pharmacodynamic (PD) analysis, pharmacokinetic (PK) analysis, biomarker analysis, and pathology and toxicity analysis in large scale. The company’s pharmacology team has developed multiple disease models, including tumor models (syngeneic models, cell line-derived xenograft (CDX) and patient-derived xenograft (PDX) models, and spontaneous models), metabolic disease models (NASH, obesity and diabetes, atherosclerosis, chronic kidney disease, fibrosis, etc.), and inflammatory and autoimmune disease models (asthma, rheumatoid arthritis, atopic dermatitis, psoriasis, SLE, MS, IBD, etc.) for preclinical drug efficacy evaluations. So far, Biocytogen has become a long-term partner for more than 400 biotech and biopharmaceutical companies around the world; more than 2000 pharmacological evaluation projects have been completed.
Animal facility:
Biocytogen has a 55,000-sqm SPF facility in Haimen (Jiangsu), China, with smaller facilities in Daxing (Beijing), China and Boston (U.S.). The total capacity is more than 150,000 cages, including 30,000 cages in 300 isolators and 120,000 cages in IVCs. The maximum supply capacity of the facility is 800,000 genetically modified mice annually. Through implementing strict scientific and standard operating procedures, and a highly efficient facility management system, the animal centers adhere to strict standards for critical quality-control testing to identify microbial contamination. Haimen Animal Center has been AAALAC accredited since 2016, and Beijing Animal Center received AAALAC accreditation in 2021; the Boston satellite facility adheres to the same standards.
With a wide range of animal strains, large-scale supply capabilities, and high-quality service capabilities, BioMice products and services have been recognized by many research institutes and biotech/biopharmaceutical companies globally. This includes almost all MNCs, leading biotech and biopharmaceutical companies in China, and many renowned research institutes, with models published in top-tier research journals. For more information about how BioMice products and services can support your research, please contact us at info@biocytogen.com.cn.
About Biocytogen
Biocytogen (HKEX: 02315) is a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies. Using its proprietary RenMab®/RenLite®/RenNano® mice platforms for fully human monoclonal, bispecific/multispecific antibody and nanobody development, Biocytogen has integrated its in vivo drug efficacy screening platforms and strong clinical development expertise to streamline the entire drug development process. Biocytogen is undertaking a large-scale project to develop first-in-class and/or best-in-class antibody drugs for more than 1000 targets, known as Project Integrum (RenMice® HiTS Platform). As of June 30, 2023, 50 therapeutic antibody co-development/out-licensing/transfer agreements and 42 target-nominated RenMice® licensing projects have been established worldwide, including several partnerships with multinational pharmaceutical companies (MNCs). Biocytogen's pipeline is comprised of 10 core assets, with partnerships established for multiple clinical assets. The company’s sub-brand, BioMice, encompasses the generation and distribution of animal model products, as well as preclinical pharmacology and gene-editing services for clients around the globe. Headquartered in Beijing, Biocytogen has branches in China (Haimen Jiangsu, Shanghai), USA (Boston, San Francisco), and Germany (Heidelberg). For more information, please visit http://en.biocytogen.com.cn.
Contacts
BioMice Website: www.biomice.com
BioMice Inquiries: info@biocytogen.com.cn
Antibody R&D Inquiries: BD-Licensing@biocytogen.com
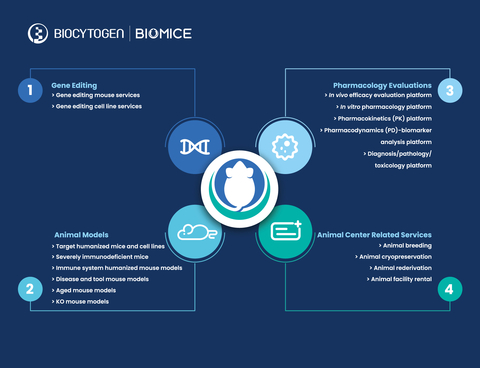
BioMice Business Summary (Graphic: Business Wire)




