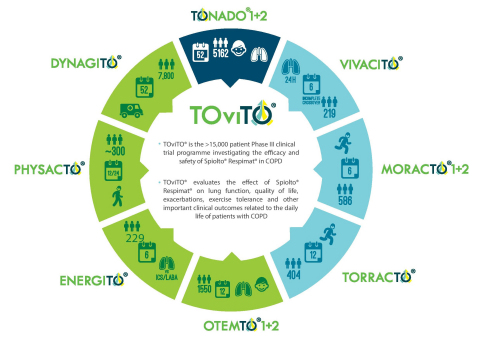
- OTEMTO® 1&2 post-hoc analysis reinforces quality of life benefits provided by Spiolto® Respimat® right from the initial stage when patients first need maintenance therapy*
- Head-to-head ENERGITO® study shows superior lung function improvements with Spiolto® Respimat® versus LABA/ICS FDC
AMSTERDAM -- (BUSINESS WIRE) -- Boehringer Ingelheim today announced new data from two trials presented at the European Respiratory Society (ERS) International Congress 2015. A post-hoc analysis from OTEMTO® 1&2 indicates that Spiolto® Respimat® provides significant quality of life improvements over Spiriva® Respimat® (tiotropium) or placebo, right from the initial stage when patients first need maintenance therapy.1 These data support the role of Spiolto® Respimat® as a new 1st line maintenance therapy for COPD†. In addition the ENERGITO® study shows that the lung function improvement§ with Spiolto® Respimat® (tiotropium/olodaterol) is superior to a steroid-containing therapy (LABA/ICS FDC‡) in patients with moderate to severe COPD.2
This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20150928005716/en/
From early in the course of COPD, decreasing lung function makes it more and more difficult for people to breathe. To cope with feeling breathless, patients reduce their activity and change their lifestyles which can lead to a ‘downward spiral’ of their condition.3 This in turn results in a large physical and emotional impact on people living with the disease.4 Providing optimal treatment right from the start of maintenance therapy can give patients the best chance of managing their symptoms, keeping active and maintaining their quality of life.
“These two trials further characterise the relevant benefits Spiolto® Respimat® provides compared to two commonly prescribed maintenance treatments: Spiriva® Respimat® and a LABA/ICS FDC,” said Dr William Mezzanotte, Vice President and Head of Respiratory Medicine at Boehringer Ingelheim. “The lung function and quality of life improvements shown in these trials add to the growing body of evidence supporting the benefit of using Spiolto® Respimat® at the initiation of maintenance therapy for COPD.”
OTEMTO® 1&2 - data reinforce Spiolto® Respimat® benefits as 1st-line maintenance therapy
A post-hoc analysis of the OTEMTO® 1&2 trials* (NCT01964352/ NCT02006732), presented at ERS for the first time, shows that Spiolto® Respimat® provides a clinically meaningful 4 point improvement in quality of life§§ compared to placebo in COPD patients with GOLD 2 disease.1 In the same patient group, 13% more patients achieved a clinically meaningful improvement in quality of life with Spiolto® Respimat® compared to Spiriva® Respimat® (52.8% compared to 39.2%).5
“With no cure for COPD, improving quality of life is one of the major goals of treatment,” said Dave Singh, Professor of clinical pharmacology and respiratory medicine, University of Manchester. “When many patients are first diagnosed their condition is already declining rapidly. Doing as much as possible, early on, can give patients the best opportunity to maintain a good quality of life for longer. This sub-analysis shows that Spiolto® Respimat® could give patients this opportunity.”
These latest results add to the recently published OTEMTO® 1&2 data in Respiratory Medicine,6 which show Spiolto® Respimat® provides consistent improvements in lung function, breathlessness and quality of life over Spiriva® Respimat® (tiotropium) or placebo. The data also shows that Spiolto® Respimat® provides a safety profile similar to Spiriva® Respimat® or placebo. Incidence of adverse events (AEs) was broadly similar across treatment groups, with a higher incidence of AEs leading to discontinuation in the placebo groups compared to the treatment groups.6
ENERGITO® - superior lung function improvements over LABA/ICS FDC
Also presented for the first time, data from ENERGITO® (NCT01969721) show that Spiolto® Respimat® met all lung function endpoints**, which included a 42% improvement in trough FEV1, compared to the twice daily LABA/ICS salmeterol/fluticasone propionate in patients with moderate to severe COPD.2
ICS, or steroid-containing therapies, can be associated with potential serious side-effects and guidelines only recommend the use of these treatments in patients with severe COPD and frequent exacerbations (more than 2 per year).7 Despite this, in clinical practice ICS-based therapies are widely used across all COPD stages even for patients with low exacerbation risk who have less severe COPD. In this respect, the results from ENERGITO® add to data from the large-scale WISDOM study,8 raising questions about whether the benefits of ICS outweigh the potential side-effects associated with this class of treatment.
“ICS-containing therapies certainly have a role in the management of COPD but questions remain regarding when to use these treatments,” said Professor Kai-Michael Beeh, Insaf GmbH Institut für Atemwegs-forschung, Wiesbaden, Germany. “The ENERGITO® results further support the potential benefit of Spiolto® Respimat® as a COPD maintenance therapy and the notion that steroid-containing therapies may only be required in a population of patients with more severe stages of the disease and frequent exacerbations.”
The OTEMTO® 1&2 and ENERGITO® trials are part of the >15,000 patient TOviTO® Phase III clinical trial programme investigating the efficacy and safety of Spiolto® Respimat® in COPD. The results build on the pivotal phase III TONADO® trials that demonstrated Spiolto® Respimat® provides significant improvements in lung function, breathlessness, quality of life and reduction in rescue medication use over Spiriva® Respimat® right from the initial disease stages when patients first need maintenance therapy.9,10 Data also showed that Spiolto® Respimat® was well tolerated with a favourable safety profile that was similar to tiotropium or olodaterol alone.9
For ‘Notes to Editors’ and ‘References’ please visit: http://www.boehringer-ingelheim.com/news/news_releases/press_releases/2015/28_september_2015_copd.html
|
_______________________ |
|
* Results are derived from a post-hoc subgroup analysis of patients with moderate COPD (GOLD stage 2). The primary endpoints of the OTEMTO® studies were: FEV1 AUC0-3, trough FEV1, SGRQ total score in patients with moderate to severe COPD (GOLD stage 2–3) † Chronic obstructive pulmonary disease § FEV1 AUC0-12 response (primary endpoint), FEV1 AUC0-24, FEV1 AUC12-24 and trough FEV1 (secondary endpoint), all measured at 6 weeks ‡ long acting beta2 agonist/inhaled corticosteroid fixed dose combination (FDC) §§ As measured by the St George’s Respiratory Questionnaire (SGRQ) ** FEV1 AUC0-12 response (primary endpoint), FEV1 AUC0-24, FEV1 AUC12-24 and trough FEV1 (secondary endpoint), all measured at 6 weeks |
|
|
![]()
View source version on businesswire.com: http://www.businesswire.com/news/home/20150928005716/en/
CONTACT:
Boehringer Ingelheim
Corporate Communications
Media + PR
Dr Kristin Jakobs
Phone: +49 6132 77 144553
Fax: +49 6132 77 6601
Email: press@boehringer-ingelheim.com
(Graphic: Business Wire)

(Photo: Business Wire)
(Photo: Business Wire)




