Precedent setting technology in Colposcopy, the current Gold Standard for detecting Cervical Cancer
SAN DIEGO--(BUSINESS WIRE)--STI Medical Systems, an industry leader in medical imaging systems and machine vision software, announced today that the U.S. Food and Drug Administration (FDA) has given clearance to the Company’s precedent-setting ImageSense™ technology for use in colposcopy. This new proprietary technology represents an entirely new way of presenting digital imagery in the doctor’s office, and is first-in-class for colposcopy. This represents an advance in colposcope system technology as colposcopes used in evaluating cervical neoplasia have remained largely unchanged for over 50 years.
Acetowhitening is one indicator currently used by doctors to identify areas of the cervix possibly containing disease. ImageSense is a tool for displaying areas of acetowhitening by producing a digital output depicting the color differences of the cervix before and after the application of acetic acid during a typical colposcopic exam. By digitizing and memorializing an otherwise transient effect and enabling on-demand viewing of the acetowhite process, ImageSense displays information to the doctor in ways previously not possible.
ImageSense technology is currently in use on the Company’s UltraSightHD™ digital colposcopy system. The combined product offers a fully integrated clinical workflow solution for colposcopy that also incorporates DICOM-compatible imagery and a seamless Electronic Medical Records (EMR) interface via its onboard touch-screen user interface. ImageSense technology provides the UltraSightHD product with a unique competitive advantage over all other colposcopes in the market.
Cervical cancer is the leading cause of death among women in developing countries. These statistics are particularly troubling since when detected early, cervical neoplasia is almost always curable. According to the WHO, there are an estimated 473,000 new cases and 253,500 deaths annually. An important reason for the sharply higher cervical cancer incidence in developing countries is the lack of effective screening programs aimed at detecting pre-cancerous conditions (dysplasia) and treating them before they progress to invasive cancer.
About STI Medical Systems
STI Medical Systems, LLC is a subsidiary of Science & Technology International, Inc, a premier Life Sciences Accelerator with a 25 year history of growth and success in developing technology with global market appeal.
STI Medical Systems is engaged in the commercialization of intelligent medical imaging systems that assist physicians in maximizing their performance and efficiency, and that improve the quality of life for patients afflicted with chronic diseases. The company has a product pipeline that will result in the release of new products in the fields of cervical cancer, colorectal cancer and diabetes management.
UltraSightHD™ and ImageSense™ are trademarks of STI Medical Systems.
Photos/Multimedia Gallery Available: http://www.businesswire.com/cgi-bin/mmg.cgi?eid=6561645&lang=en
Contacts
For press information:
STI Medical Systems, LLC
Will Alameida, 808-540-4778
Executive Vice President
will@sti-hawaii.com
www.UltraSightHD.com
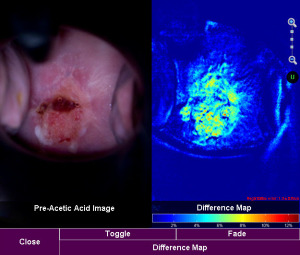
ImageSense technology displays areas of aceto-whitening, one indicator of possible cervical disease. (Photo: Business Wire)




