Bifidobacterium longum BB536, a clinically proven multifunctional probiotic strain that was originated from the gut of a healthy breast-fed infant in 1969, is celebrating its 50th anniversary of its discovery in 2019.
The probiotic strain Bifidobacterium longum BB536 had previously attained official confirmation from the U.S. Food and Drug Administration (FDA) and was recognized as safe and suitable for use in a variety of food products and dietary supplements (GRAS Notice No. GRN 000268). With this newest self-affirmed GRAS status, Morinaga Milk is now expanding the opportunities of its flagship probiotic strain BB536 into the new space – to be specifically used in infant formulas.
Following a comprehensive and critical evaluation of the safety of B. longum BB536, the strain was deemed Generally Recognized As Safe (GRAS) for use as a probiotic ingredient in infant formula. The evaluation was conducted by an independent panel of qualified scientific experts, advised by Claire Kruger, Ph.D., D.A.B.T., president of Rockville, MD-based Spherix Consulting, to ensure that BB536 meets the highest standards of safety and regulatory compliance. The company said it will submit a GRAS Notification for BB536 to the FDA.
“This incredible achievement is yet another indication of our commitment to continuously strive to add value to our flagship probiotic strain, the foundation of which lies in safety and efficacy,” said Ko Shiino, General Manager of Sales and Marketing Department, International Division of Morinaga Milk. “The new GRAS status is also a stepping stone in our efforts to further backing the scientific rigor of our proprietary probiotic strain. We are extremely delighted that BB536 has passed the stringent process to obtain the new GRAS status pertaining to infants,” he added. Further, the potential implications of this new status are very exciting to be a part of.
First Among Many: Publication of A Strain-Specific Scientific Review
Embracing a major milestone, Morinaga Milk is also proud to announce the publication of a scientific review on the clinical efficacy and safety of its flagship probiotic strain BB536. In tandem with the 50th anniversary of the discovery of BB536, the publication of the review (Wong et al., 2019) in Journal of Functional Foods provides a comprehensive overview on the huge amount of scientific information available regarding health benefits, safety, and mechanism of actions of BB536 as a multifunctional human probiotic.
“We are extremely honored to have BB536 to stand out from the many undocumented strains being marketed speciously as probiotics. This scientific review would serve as a fundamental framework to better understand the clinical effectiveness of BB536 and how such beneficial effects take place,” said Dr. Xiao, General Manager of Next Generation Science Institute of Morinaga Milk. “We strongly believe that the publication of this scientific review can provide both medical care professionals and the public more reliable health claims in helping them to make better-informed probiotics selection,” he noted.
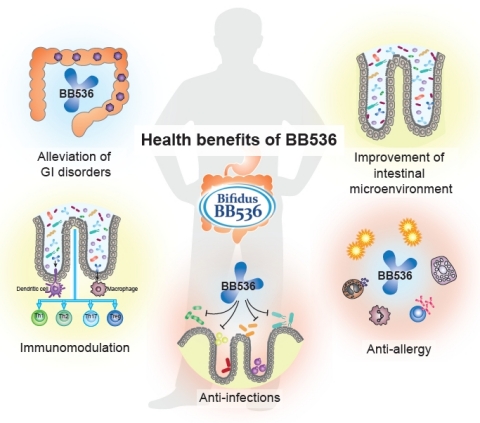
BB536 Celebrates 50 Years of Empowering Better Health
BB536 is a clinically effective, well-established, multifunctional probiotic strain that has a long history of human use in alleviating gastrointestinal, immunological and infectious diseases. For half a century, BB536 has been the superior Human-Residential Bifidobacteria (HRB) probiotic strain that helps people achieve optimal health from the inside out. Ingestion of BB536 can provide a consistent beneficial effect in improvement of gastrointestinal conditions, maintenance of intestinal microflora balance, regulation of immune response, anti-allergy, and protection against microbial infections. It is evidenced that BB536 acts in concert with the gut microbiota to drive a fine-tuned intestinal and immune balance.
BB536 has been used as a functional food ingredient in various products such as milk-based drink, yogurt, infant formula, and nutritional supplements and has been marketed in over 30 countries for more than 40 years. “In fact, BB536 is considered as one of the best characterized probiotic and has gained importance thanks to its substantial beneficial effects in the treatment and management of human health without any adverse effects,” the company adds.
Backed by Human Substantiation, Safety and Efficacy
“Along with an exceptionally long history of safe use in humans, as published in the review, the safety and efficacy of BB536 continues to be undoubtedly proven through solid science, leading to the receipt of self-affirmed GRAS approval,” said Dr. Abe, Corporate Officer and General Manager of Food Ingredients & Technology Institute of Morinaga Milk.
In recent years, probiotics have become increasingly popular in infant products as awareness continue to rise around the importance of stimulating beneficial bacteria from the beginning of life for prevention against various health-related complications. However, as the use and diversity of probiotic products expand, choosing an appropriate type of probiotic has been challenging due to differences in the mechanisms of action, safety profile, origin, and efficacy of different strains. Questions and concerns have been raised about the safety and clinical efficacy of probiotic administration, especially if the product is destined for use in infants.
“Affirmation of this important self-affirmed GRAS status confirms that the science behind the probiotic strain BB536 is sufficient to guarantee its safety in infant formula. This greatly allows us to diversify the application options available to our customers,” he added.
About Morinaga
Morinaga Milk Industry Co., Ltd. is one of the leading dairy product companies in Japan with a century of history harnessing the nutritional properties of dairy products and its functional ingredients. Morinaga Milk excels in innovative technology and offers various dairy products and other beneficial functional ingredients to customers throughout the world. Since 1960s, Morinaga Milk has been engaged in research on the safety, functional health benefits, and mechanisms of action of probiotic bifidobacteria to better understand their role in maintaining human health. For more information about Morinaga Bifidobacteria, please visit us at http://bb536.jp/english/index.html.